Therapeutic solutions for more evolved care
Over the years, our Researchers have developed innovative technologies, novel formulations and delivery systems better suited to care.
Over the years, our Researchers have developed innovative technologies, novel formulations and delivery systems better suited to care.
Given the demanding challenge that the medically-assisted procreation (MAP) area represents for the pharmaceutical sector, IBSA has patented exclusive extraction and purification processes and installed equipment dedicated to the production of fertility hormones, gonadotropins, using the urine of pregnant and post-menopausal donors. Gonadotropins are glycoprotein hormones composed of two non-covalently associated protein subunits - the alpha subunit and the beta subunit. The alpha subunit contains 92 amino acids (AA) and is identical in FSH (follicle–stimulating hormone), LH (luteinising hormone) and HCG (human chorionic gonadotropin). The beta subunits, on the other hand, differ and confer receptor specificity and different biological properties. The extent and characteristics of the post-translational changes of these molecules give different charges, biological activities and half-lives to each type of glycoprotein.
The fertility hormone range is produced in compliance with the highest purity, efficacy and safety standards, whilst keeping the structure of the glycoproteins intact.
Combining advanced technologies with a full awareness of the relationship between the structure and function of gonadotropins has made the processes patented by IBSA a quality benchmark.
This purification process consists of two steps:
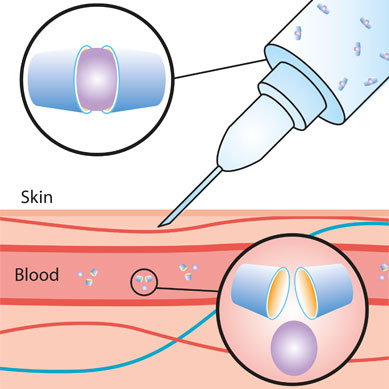
IBSA’s research and development has allowed it to place on the market the only progesterone formulation in an aqueous solution, which can be administered subcutaneously.
This new formulation is used for luteal phase support in Artificial Reproductive Techniques programmes.
The brainwave that resulted in IBSA developing progesterone for subcutaneous administration was to use a new highly-purified hydroxypropyl-β-cyclodextrin (HPBCD), to increase the solubility of progesterone by exploiting the chemical properties of these molecules. The research conducted by IBSA had shown that the HPBCD products available on the market were not of a purity such as to allow the stability of the HPBCD–progesterone solution. IBSA, in conjunction with Roquette, developed and synthesised a new high-purity HPBCD.
From a chemical standpoint, hydroxypropyl-β-cyclodextrins are cyclic oligosaccharides with an outer hydrophilic part and a lipophilic core that are able to form a complex with hydrophobic drugs, thereby increasing their water solubility.
The resulting complex is readily absorbable and can be administered via the subcutaneous route, making administration more patient-friendly than vaginal and intramuscular preparations.
As progesterone is not water-soluble, most of the preparations available on the market are oil-based (ethyl oleate, seed or peanut oil).
As it is a hydrophobic molecule, until now it could not be administered subcutaneously or intravenously.
The new water-soluble progesterone formulation patented by IBSA, on the other hand, can be administered via the subcutaneous route, thus facilitating patient compliance.
Once it has been injected and absorbed, the progesterone molecule immediately breaks away from the complex with hydroxypropyl-β-cyclodextrin and is released into the bloodstream, as though the hormone had been produced physiologically by the corpus luteum.
Polyolefin plastic single-dose units are a common primary packaging solution that is often used for the administration of single-use liquid products (typical of the ophthalmology sector). The IBSA innovation consisted in using this delivery system to guarantee the release of precise doses of medicinal products for oral use dissolved in a pre-set volume. Thyroid hormones (T4 and T3) are highly active molecules that require an exact therapeutic dose; however, with multidose delivery systems, the dose is managed by means of the number of drops, which results in inaccuracy in the administration of the prescribed dose. The use of single-dose containers also makes it possible to provide the patient with the exact dose, ready to use at the dosage prescribed by the specialist, and avoids the need for an excessive use of preservatives, which are indispensable for multidose formulations.
During manufacture, once it has been prepared in a dissolver tank, the pharmaceutical solution is dispensed using special filling and sealing machines that, starting with an open, empty strip, make it possible to obtain the finished product ready to be packaged in the aforesaid sachet and then in the outer box. Single-dose strips make it possible to obtain pre-set doses, which are indicated on a colour-coded label providing all the necessary information. This label can be applied to the removable part of each single-dose container, making it possible to prevent it from coming into direct contact with the wall of the container, which could cause contamination by its adhesive substances.
The decision to use this type of packaging for certain medicinal products was based on preliminary studies on the compatibility between the ingredients of the solution and different plastic materials, in order to select the best container closure system. The resulting choice was a low-density polyethylene (LDPE) polymer, which is considered to be a good compromise also in terms of softness, which is crucial for the final elasticity of the container. Appropriate studies of the leachables and extractables (contaminants) were also conducted to determine the presence of contaminants that could have been released during contact with the packaging materials.
In literature, injection-moulded LDPE devices are described as semi-permeable containers due to their porous nature that allows volatile chemical products, i.e. substances with a moderate to high steam pressure, to permeate the container. The airtight secondary packaging was added to prevent this from happening.
Soft capsules were invented in the 1930s to conceal the unpleasant taste and smell of medicines. Since then, production techniques have greatly improved. It has been estimated that over 40% of active pharmaceutical ingredients (APIs) have poor biopharmaceutical properties, such as poor water-solubility and/or permeability. These characteristics are somewhat problematic for the oral bioavailability of compounds that need to be formulated as medicines that are bioavailable when taken by the oral route.
The oral administration of certain medicinal products as solid pharmaceutical forms (e.g. tablets) is a technologically arduous challenge, because certain active ingredients can be oily or poorly soluble in water.
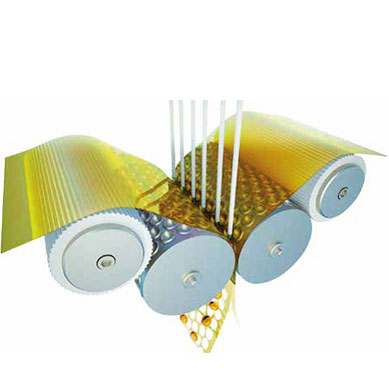
PEARLtec technology is a process for obtaining softgel (soft capsules) that makes it possible for a liquid matrix, in a suspension or a gel, to be incorporated into a continuous soft gelatine shell, thereby improving both the oral intake of the medicinal product and patient compliance.
The first step consists in weighing the capsule ingredients, before they are processed using dedicated machines known as turbo emulsifiers. During this part of the process, the optimally balanced excipients are blended together and prepared for the next step.
The heart of the process is the encapsulation step (PEARLtec). The mass that will form the capsule shell is hot-extruded into two ribbons that pass through two roller moulds positioned opposite one another, and that give the capsule its shape. A high-precision syringe pump then injects the previously formulated active substance, inside a mass known as filler, into the capsule being moulded.
This makes it possible to obtain tiny transparent beads consisting of two heat-moulded shells.
During the formation of the capsule, all the essential and critical parameters are monitored by means of in-process controls. This makes it possible to keep the quality of the finished product under control.
Before packaging, the dried capsules are inspected one by one in order to eliminate any flaws.
The quality and stability of the finished product are further guaranteed by special primary and secondary packaging.
In short, this technology makes it possible to take a liquid solution in a solid pharmaceutical form, which is highly advantageous for very low concentration formulations, as it ensures dose uniformity.
Hyaluronic acid (HA) is a substance that is produced naturally by the human body, in which it is found in the highest concentrations in the dermis and synovial fluid. It serves important functions, such as lubricating and protecting the joint cartilage and hydrating the skin and mucous membranes. The presence of hyaluronic acid decreases physiologically with age or in the presence of certain medical conditions, such as joint diseases. Consequently, in certain situations it becomes very useful to restore an adequate concentration of HA in the tissues, by means of hyaluronic acid injections. When the injection is made into a large or small joint, the treatment is known as viscosupplementation. This is because the hyaluronic acid present in the synovial fluid gives it the characteristics of viscosity and elasticity required to serve its main functions: lubrication, at rest, and protection (shock absorption), during movement. When the concentration of HA in the synovial fluid drops, due either to age or disease, the fluid loses some of its viscosity and elasticity. Hyaluronic acid supplementation by injection makes it possible to replenish the concentration of the molecule, thereby restoring the synovial fluid’s correct function.
The effectiveness of the viscosupplementation treatment depends on the extent to which the characteristics of the solution of hyaluronic acid administered mimic the natural characteristics of synovial fluid. With this in mind, IBSA developed and patented NAHYCO® HYBRID TECHNOLOGY, which makes it possible to deliver high doses of hyaluronic acid, thereby improving the viscous and elastic properties of the formulation and improving its resistance to degradation. This technology uses polymer chains of various lengths (i.e. different molecular weights): by means of a heat treatment, high-molecular-weight hyaluronic acid (H-HA) and low-molecular-weight hyaluronic acid (L-HA) form strong hydrogen bonds to form hybrid cooperative complexes without using chemical cross-linking agents. This makes it possible to achieve high concentrations of HA without prejudice to the ease and safety of injection and effectively mimicking the physical and mechanical properties of healthy synovial fluid, thereby optimising the concept of viscosupplementation.
The same NAHYCO technology can also be used with success in other indications. It can be used, for example, to replenish the physiological concentration of HA in the skin, improving its water content and consequently the appearance of the ageing dermis. Once again, the possibility of obtaining high concentrations and optimised characteristics allows straight-forward delivery of high doses of HA that distribute within the dermis to achieve an optimum result.
At the same time, the technology guarantees absolute safety, due to the use of high-purity hyaluronic acid, obtained by fermentation rather than extraction from animals, and that is identical to that present in the human body, without requiring any chemical modification.
It should also be emphasised that although the NAHYCO technology applies primarily to hyaluronic acid, it can also be extended to mixtures of high-molecular-weight hyaluronic acid and other polymers of the same family (glycosaminoglycans, or GAGs, with include chondroitin). The hybrid cooperative complex can therefore be formed, for example, by combining high-molecular-weight HA with biotechnological sodium chondroitin sulphate, where the sodium chondroitin sulphate, which IBSA obtains by fermentation using a patented process, serves the same function as low-molecular-weight hyaluronic acid.
IBSA possesses the technological know-how to develop and produce in its plants single- and multilayer medicated patches designed to act either locally or systemically, depending on the site of action of the active substance. Both types, which are produced using Hydrogel and Drug-in-adhesive technologies, are patent-protected, and this know-how sets IBSA apart in the European pharmaceutical sector, as one of the few companies in the world to be able to offer both.
The structure of these patches is usually composed of a polyacrylate, silicone or other adhesive matrix containing the active substance, a support layer and a protective layer to be removed before application; it can also be more complex, for example, include multiple layers of adhesive containing different concentrations of the active substance.
Patches for topical use can be either formulated to have a local action over a limited area (e.g. diclofenac and piroxicam) or transdermal, with a systemic action (e.g. nitro-glycerine and fentanyl).
Patches for topical use are able to release a medicinal product in a controlled manner, over periods of time that vary from a few hours to a week, offering a great many advantages over oral administration, such as: maintenance of optimum concentrations of the medicinal product; reduction in the frequency of administration; better tolerability.
Lastly, use of patches eliminates the hepatic first-pass effect that occurs with oral administration and, in general, makes it possible to optimise bioavailability and reduce side effects.
The field of application ranges from patches with anti-inflammatory activity to those with dermatological activity.
IBSA currently places on the market patches containing both locally- and systemically-released diclofenac, fentanyl, nitro-glycerine and piroxicam. We are also developing products for application in other therapeutic areas including the cardiovascular and dermatological fields, neurodegenerative diseases and pain therapy.
Its vast technological know-how and productive capacity make IBSA one of the world’s leading manufacturers of topical patches, a sector that the company has innovated by filing a number of patents.
A great many pharmaceutical, medical and personal hygiene products take the form of semisolids (creams and gels) or liquids in sprays (aerosols). The packaging traditionally used for these forms are flexible aluminium or plastic tubes for creams and cans with propellants or a pump dispenser for liquid sprays.
In order to overcome some of the limits of conventional packaging and to obtain a more versatile solution suited to diverse products, IBSA has introduced into its plants the Bag-on-valve (BoV) packaging technology. In this type of packaging, the product, be it solid or liquid, is placed inside a protective bag, consisting in a laminated, heat-sealed multiply (aluminium and plastic) bag. This bag, the top of which is fitted with a dispenser valve, is contained inside an aluminium can. The system is completed by a dispenser button that, when pressed, activates the valve to release the product inside the bag.
In conventional packs, the product is dispensed due to the force applied by the user, who squeezes the tube of cream or presses the dispenser pump on the spray can. Alternatively, in the case of pressurised aerosol cans, the product is dispensed thanks to its being mixed with a compressed gas: when the user presses the button, the gas is released, taking with it the product and creating the spray.
In BoV packaging, the force that is exploited to dispense the product is again a compressed gas; however, in this case, the gas is not mixed with the product, rather it is pressurised into the space between the bag containing the product and the can containing the bag. This affords a number of advantages over conventional packaging, tubes or propellent sprays: the product does not come into contact with the pressurised gas; as it is not necessary to modify the formulation, BoV technology is far more versatile and can be used not only to package liquids (sprays), but also creams and gels. Product dispensing is practical, uniform, complete (>99%) and possible in any position (360°). There is a reduction in the environmental impact, because the compressed gas is air or nitrogen and it is not released into the atmosphere during dispensing, which also prevents the cooling of the product that is associated with propellent sprays; the type of gas used, which is harmless and non-flammable, also makes BoV packaging safer. The product contained inside the BoV bag never comes into contact with the exterior, even after dispensing. Therefore, compared to a conventional tube, it affords better protection, for example against atmospheric oxidation, longer stability and lesser need for preservatives. In the case of sterile preparations, sterility is preserved even after the first dispensing.
On account of its versatility and advantages, IBSA uses BoV packaging technology to manufacture medicinal products, medical devices and cosmetics in both cream or gel and liquid form.
Orodispersible films (ODF), also known as orosoluble films or simply oral films, are a new oral pharmaceutical form, whose characteristics are able to improve the treatment compliance of certain groups of subjects whose needs are not met by capsules and tablets. They take the form of small, thin, flexible sheets, similar to postage stamps that, when placed inside the mouth, dissolve rapidly in contact with saliva. The dissolution time is usually a few dozen seconds and in any case no more than one minute. The weight of a single film is usually a few hundred mg, meaning that very little saliva is required for dissolution. ODFs are normally individually-packaged in heat-sealed sachets, in order to preserve their mechanical properties, avoid contact with atmospheric humidity and guarantee adequate stability. Individual packaging, combined with a limited size and weight make the individual dose unit convenient and discrete, even for use outside the home, in all situations, because it does not need to be taken with water. The use of ODFs is still limited to just a few applications, due to their complex manufacturing process (very limited number of companies are able to master this technology on an industrial scale).
The main production technique for ODFs is based on the coating process. In this case, the first step of production is the preparation of a homogeneous coating mass in which all the ingredients are dissolved in a suitable solvent, usually it’s water – it’s possible to incorporate other poorly-soluble active substances or functional ingredients into this mass, provided they are homogeneously dispersed. In order to achieve this aim, it is necessary to act both on the particle size of these insoluble ingredients, which must be suitably fine and poorly dispersed, and on the dissolver tank stirring system in which the mass is prepared, which can be also fitted with a turbo emulsifier. The coating mass is then used to feed a continuous coating machine inside which, using a calibrated blade, it is distributed evenly over a ribbon of plastic medium (liner) to obtain a layer with an even, pre-set thickness. This then passes through a heated tunnel, where the water is made to evaporate under controlled conditions until the orodispersible film takes on its definitive form. The last step of the process involves cutting the film down to single dose unit size, removal of the supporting liner and individual packaging in a heat-sealed sachet of compound material. The ODFs obtained by coating are usually square or rectangular, with a thickness of a few hundred microns and sides with lengths that can vary from 1 to 4 cm. A correct coating process makes it possible to guarantee a uniform content in line with the pharmaceutical requirements, ensuring that the concentration of active ingredients is constant over the entire area of the film. Thanks to the water-soluble film-forming polymer that constitutes the main structure of the film, the ODF has good tensile strength, elasticity and flexibility. These properties give the ODF good manageability, which is extremely important for the user, and appropriate processability, which is essential for large-scale production. A film-forming polymer and a plasticiser alone could be sufficient to obtain an ODF; however, these two ingredients are usually combined with a flavouring, to improve palatability, and a colouring, to improve its appearance. As mentioned previously, the main ingredient of an ODF is the film-forming polymer, which can also be a blend of two or more polymers. There are a number of water-soluble polymers that can serve this purpose, including pullulans, alginates, modified cellulose and maltodextrins. It is important for the polymer to have not just the right solubility, processability, manageability and stability characteristics, but also that is has a neutral or pleasant flavour and does not leave residues in the mouth.
For the production of its orodispersible films, IBSA chose maltodextrins as the main polymer, taking to industrial scale a patented formulation platform that forms the basis of the IBSA FilmTecTM technology. Maltodextrins have the advantage of being a common, affordable food ingredient with good palatability and rapid dissolution times inside the mouth, where they dissolve completely without leaving an aftertaste.
Orodispersible films always permit immediate release: ODFs release their ingredients very rapidly and can therefore make their absorption easier and quicker than with a tablet or capsule. In certain specific cases, depending on the nature of the active substance, ODFs can also improve the absorption of active substances and functional ingredients.
Orosoluble preparations such as ODFs are the preferred oral form for most people. For certain user categories, such as dysphagic, bed-ridden and elderly subjects, those who have problems taking water and children, the use of ODFs instead of conventional oral forms becomes a necessity rather than a mere preference. As a matter of fact, when compared to tablets, capsules, powders and syrups, orodispersible films allow a precise and accurate dose even for those who have problems swallowing or cannot use water to aid administration.
The manufacture of medicinal products and medical devices often involves the use of ampoules and vials containing ready-to-use solution or powders to be dissolved at the time of the injection. This type of packaging ensures good product protection, but also makes its administration rather complex. The liquid (or powder after dissolution) must be drawn out using a syringe, by piercing the membrane on the vial or snapping the tip off the glass ampoule, operations that require both dexterity and experience. Self-administration is difficult, and the risk of dosing errors and contamination relatively high.
The use of syringe vials, i.e. syringes pre-filled with the right volume of product, greatly simplifies the administration of sterile products for injection and reduces the risk of errors, consequently improving safety. The ease of use favours self-administration and use in emergency situations, in which the injection must be performed as rapidly as possible. It avoids the risk of bubbles forming in the syringe and the risk of contamination, which are always present when products are drawn from ampoules or vials. Pre-filled syringes guarantee a certain dose, with complete product recovery, and ensure sterility.
IBSA production lines are able to manufacture sterile pre-filled syringes with different capacities, formats and materials. The presence of highly-automated lines make it possible to achieve high production volumes, whereas the predominant use of glass syringes, combined with a rigorous control of product interactions with the packaging, makes it possible to guarantee the quality and stability of medicinal products and medical devices for injection.
Sterility is obtained by processing under aseptic conditions or by terminal sterilisation. More specifically, terminal sterilisation in an autoclave is the process of election for high-viscosity solutions. For this type of product, conventional packaging in ampoules and vials is even more disadvantageous and the use of pre-filled syringes is the best technological solution. IBSA has therefore installed lines dedicated to the manufacture and filling into pre-filled syringes of high-viscosity solutions, such as hyaluronic acid solutions for intraarticular and intradermal injection.
Device for intravesical glycosaminoglycan (GAG) infusion
The urothelium is a type of epithelial tissue that lines the bladder and the urinary tract. The urothelium is covered by a layer of polyanionic molecules constituted primarily by glycosaminoglycans (GAG), a class of sugars that form a protective, neutralising barrier that is impermeable to both toxic substances and microorganisms. Of the various GAGs making up this barrier, hyaluronic acid and chondroitin sulphate play a key role in its function. Qualitative and quantitative variations in these two GAGs at different levels eliminate the barrier effect, generating a series of conditions that can favour the onset of cystitis of various types (e.g. interstitial cystitis, recurrent cystitis caused by infection, etc.). Hyaluronic acid (HA) and chondroitin sulphate (CS) are often used for these therapeutic purposes in the form of a very diluted solution of the individual GAGs, delivered using a catheter. The relatively low sodium hyaluronate content is made possible by its physical and chemical characteristics and its aqueous solutions present an excessive increase in viscosity with concentration. Therefore, an indiscriminate increase in the concentration of the active substance (despite its excellent water-solubility) is not feasible for the therapeutic purposes considered here, because the consequent substantial increase in viscosity would make the administration of the solution by catheter difficult and painful. It is also known that combining hyaluronic acid with chondroitin sulphate produces an excessive increase in viscosity due to the two molecules’ propensity to clump together 1,2. IBSA research found a surprising solution to this problem by discovering that hyaluronic acid can be combined with chondroitin sulphate using bivalent metal ions that act by reducing the viscosity of the solution.
The product developed by IBSA consists in a 50-mL Crystal Clear pre-filled syringes (1.6% HA, 2% CS, 0.87% CaCl2) and a novel patented medical device (IALUADAPTER®) that makes it possible to inject a glycosaminoglycan solution into the bladder using a minimally-invasive procedure without requiring catheterisation. IALUADAPTER® can be an alternative to the use of standard male and female catheters and its main advantage is that it eliminates the pain associated with the catheterisation procedure.
As, using IALUADAPTER®, the solution passes straight into the bladder through the urethra, it simultaneously treats both the urethral and the vesical mucosae. IALUADAPTER® is designed to fit snugly against the opening of the urethra, to allow the fluid to flow into the bladder without leakage of the solution. The device consists of four main parts:
Back to top